Insulin is the first-line treatment for Type 1 diabetes and the final mode of treatment for Type 2 diabetes after oral antidiabetic options have been exhausted. Insulin serves two purposes in our body. At normal levels, it functions as an anabolic hormone, allowing the body to store carbohydrates, fats, and proteins. However, at very low levels, insulin acts as an anti-catabolic hormone. Type 1 diabetics are usually thin, undernourished, experience failure to thrive, and may suffer from weight loss due to the lack of sufficient insulin.
When insulin levels fall below the critical threshold needed to maintain normal body functions, there is increased breakdown of fats and proteins, leading to the presence of ketone bodies in the urine. Even a small amount of insulin in the body can prevent this complication. Therefore, it is crucial to check for ketones in the urine of patients with poor glycemic control and failure to thrive.
Patients with no systemic symptoms and 1+ ketones in the urine may still be managed with oral hypoglycemic drugs like sulfonylureas and DPP4 inhibitors. However, patients presenting with ketoacidosis require insulin.
How to Initiate Insulin in a Patient:
A patient may present to you in the outpatient department (OPD) or the emergency department. OPD management usually starts with long-acting insulins (which have a duration of action of approximately 24 hours and are given once a day, e.g., Glargine, Detemir, Degludec). These insulins control fasting blood sugar levels. Emergency management involves the use of fast-acting insulins (which have a duration of action of 4-6 hours and are given before every meal). These insulins control postprandial blood sugar levels.
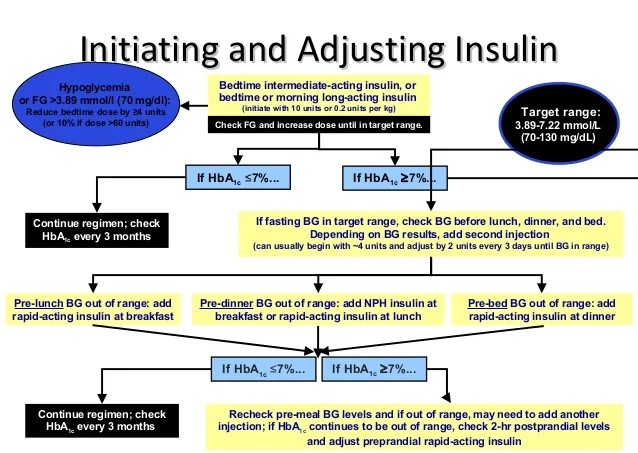
Initiation of Long-Acting Insulin:
Long-acting insulins are usually administered subcutaneously once a day and have a very good safety profile. They need to be administered at the same time every day, and meal intake has little impact on them. Their main role is to prevent catabolism. After a patient is diagnosed with Type 1 diabetes or has exhausted the options of hypoglycemic drugs in Type 2 diabetes (persistently elevated blood sugar levels, high pill burden, and ketonuria), insulin may be initiated along with other medications.
A rough calculation of the insulin-to-blood sugar ratio is that 2 units of insulin lower blood sugar by about 50 mg/dL. However, several factors can impact this ratio, such as insulin resistance, glucotoxicity, and basal insulin requirements. We will discuss each of these factors individually.
Insulin Resistance is usually the result of hyperglycemic hormones like cortisol, adrenaline, and glucagon. These hormones, typically secreted in response to physical or emotional stress, combined with a lack of rest, lead to elevated blood sugar levels, which exhaust the pancreas’s ability to secrete sufficient insulin.
Glucotoxicity refers to the initial resistance to sugar-lowering effects due to long-term elevated blood sugar levels. This condition gradually improves as blood sugar levels decrease, necessitating a reduction in insulin dosage.
Basal Insulin Requirement is, as we discussed earlier, the minimum dose required to prevent catabolism. It depends on the body’s basal metabolic rate, which can increase during medical conditions such as surgery, trauma, or stress, as well as the amount of insulin naturally produced by the body.
Considering these factors, we start with a long-acting insulin dose calculated based on the number of units needed to bring fasting blood sugar levels closer to normal. Overzealous correction should be avoided, as patients may develop signs of hypoglycemia even at elevated fasting blood sugar levels. A gradual correction is recommended, with an initial target of maintaining blood sugar levels around 120-130 mg/dL.
For example, if a patient’s fasting blood sugar level is 224 mg/dL (100 mg/dL above the desired level), we might start with 4 units of long-acting insulin (since 2 units of insulin lower blood sugar by 50 mg/dL). However, this may not be sufficient due to the factors mentioned above (insulin resistance, glucotoxicity, and basal insulin requirement). Therefore, experienced physicians may begin insulin at a higher dose. For less experienced physicians, it is recommended to adjust the insulin dose by 2 units every other day. It is crucial to monitor fasting blood sugar levels daily until a stable level is achieved.
So, let’s say that after administering 4 units of insulin for 2 days, the fasting blood sugar levels drop to 176 mg/dL. We then increase the dose by another 2 units, bringing it to 6 units for 2 days. After this adjustment, the blood sugar levels decrease to 138 mg/dL. At this point, you might be unsure whether to increase the dose by 1 or 2 units. Always opt to go slow. By increasing the insulin dose by one unit (to a total of 7 units), we achieve a blood sugar level of 122 mg/dL. This should be accepted as the baseline dose, with the expectation that as glucotoxicity improves and the patient’s general condition (including basal metabolic rate and stress hormone levels) stabilizes, fasting blood sugar levels will also improve.
In summary, we have achieved fasting blood sugar control within a week of initiating insulin.
